Description
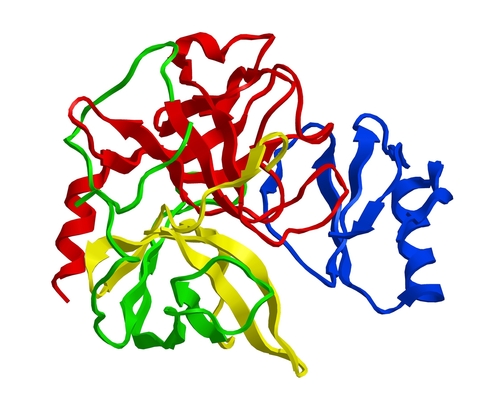
Chymotrypsin is a serine protease isolated from bovine pancreatic extracts, which preferentially catalyzes the hydrolysis of peptide bonds involving L-isomers of tyrosine, phenylalanine and tryptophan. It also readily acts upon amides and esters of susceptible amino acids. Chymotrypsin catalyzes the hydrolysis of bonds of leucyl, methionyl, asparaginyl and glutamyl residues. The selectivity of this enzyme is important for reproducible protein digestion and mass spectrometry (MS)-based protein identification.
When compared to trypsin, chymotrypsin associated digestion typically generates a large number of shorter peptides. The endoproteinase chymotrypsin specifically cleaves at the carboxyl side of tyrosine, phenylalanine, tryptophan and leucine. Two very similar (80% homology) and predominant forms of chymotrypsin, A and B, are found in equal amounts in bovine pancreas, however, they have different proteolytic characteristics. Chymotrypsin can tolerate mild denaturing conditions, such as 0.1% SDS, 2M urea, 2M guanidine·HCl, 1% CHAPS, and 30% acetonitrile with optimal enzymatic activity.
Molecular Weight: 25 kDa
pH: 7.5 to 8.5
Form: Lyophilized powder
Storage temp.: -20°C, stable for 1 year
Additional information
| Size | 1 x 25 µg, 2 x 25 µg, 4 x 25 µg |
|---|

![Tris-Glycine-SDS Running Buffer [10X]](https://www.cephamls.com/wp-content/uploads/2019/02/TRIS-G3-1-scaled-350x467.jpg)

